Special Feature An FFC that Improves the Skin’s Barrier Function from the Inside Out (PHYCONA Skin Moistlifting Tablets)

Value Creation Edible skin care: A new approach to increasing skin’s moisture retention
DIC’s new PHYCONA supplement offers a safe alternative for individuals who feel that there are limits to the benefits of topical skin-care products.
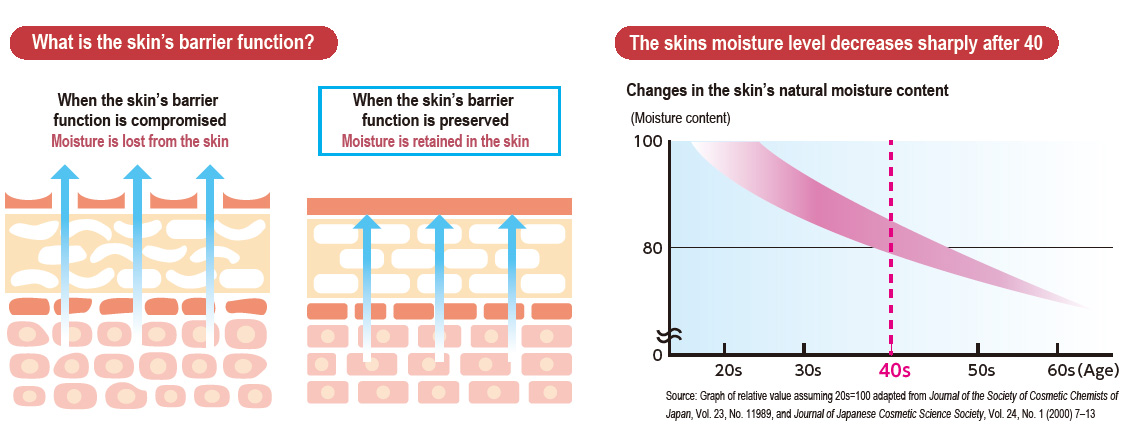
Youthful and healthy skin is the earnest desire of many people— women in particular. However, the reality is that most people see their skin begin to lose moisture in their 40s. This is because the skin’s barrier function, which prevents moisture in the skin from evaporating, deteriorates with age. When the skin’s barrier function is compromised, whether due to age, irregular lifestyle or poor nutrition, skin becomes dry. For this reason, many people feel that there are limits to the benefits of topical skin-care products and that dry skin is the main problem they face regardless of the season. The DIC Group, which seeks to support healthy and comfortable lifestyles, has applied its experience in the cultivation of Spirulina, which is already used widely in dietary supplements and food products, recognizing the potential of this edible nutrient-rich bluegreen algae to improve skin’s moisture retention.
Developing PHYCONA, a skin-care supplement that enhances the skin’s barrier function from the inside out
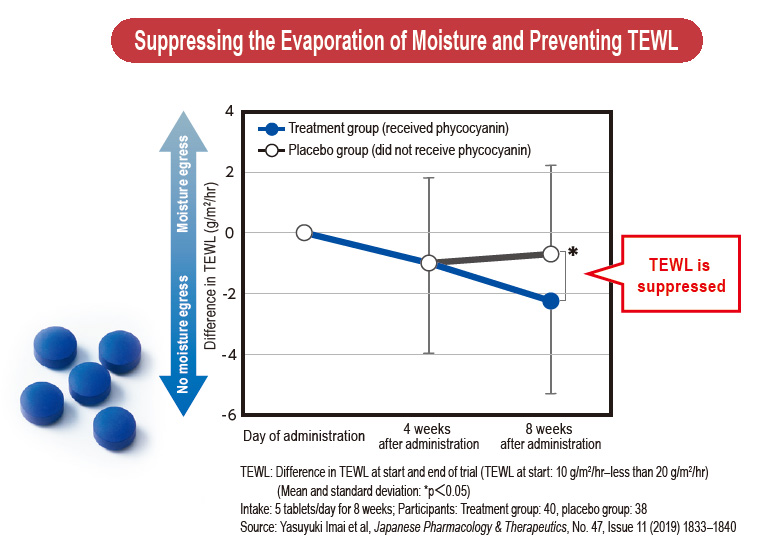
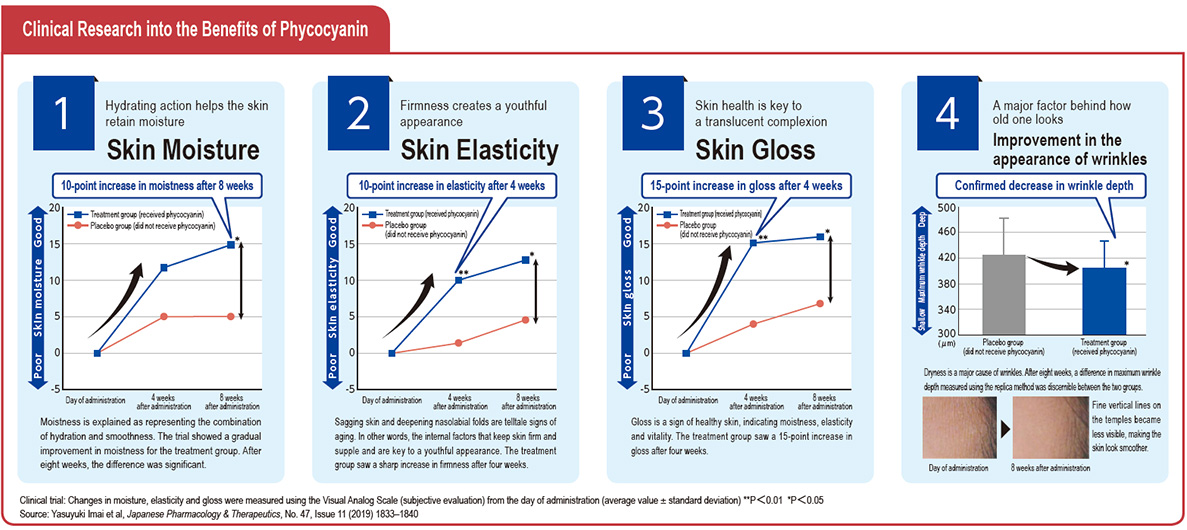
Focusing on the action on the skin of phycocyanin, a pigment–protein complex found in Spirulina, when consumed, DIC found that it improves the skin’s barrier function, which suppresses transepidermal water loss (TEWL). Capitalizing on its patented high-yield extraction technologies for high-grade C-phycocyanin and allophycocyanin from Spirulina, DIC succeeded in commercializing a new skin-care supplement, dubbed PHYCONA. In a world first, the Company also conducted a clinical trial to verify the effects of this new supplement as a food product with phycocyanin as an ingredient, scientifically demonstrating its ability to enhance the skin’s moisture retention. As a consequence, an application to have PHYCONA approved as the first Spirulina-derived Food with Functional Claims (FFC) was accepted and in October 2020 DIC launched PHYCONA Skin Moistlifting tablets.
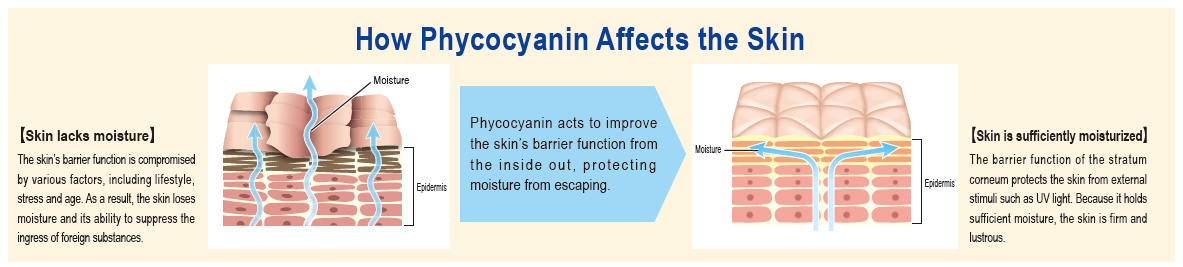
PHYCONA’s principal raw material is Spirulina, which DIC has cultivated for half a century
In the 1960s, amid concerns that rapid population growth worldwide would lead to a
food crisis, researchers actively explored the production of food-grade yeast by feeding
it petroleum byproducts. Against this backdrop, the efforts of DIC, which entered the
biochemicals business with the objective of developing new sources of protein, led it to
Spirulina, an edible blue-green algae containing more than 50 nutrients and other healthboosting
constituents, including amino acids, vitamins and minerals, that grows naturally
in many tropical regions of the world. DIC commenced research into the cultivation of
Spirulina in 1970 and in 1977 became the world’s first successful commercial mass
producer of the algae.
In subsequent years, DIC established the world’s largest Spirulina farms in the United
States and the PRC, where it produces high-grade Spirulina under quality control systems
that satisfy stringent global standards. In addition to nutrients and other health-boosting
constituents, the Company also extracts natural colorants made with phycocyanin, a blue pigment found in the algae. In 1999, DIC established wholly owned subsidiary DIC Lifetec
to further strengthen its production and sale of Spirulina-based products.
Today, Spirulina—commonly referred to as “the king of superfoods” thanks to its
superb nutritional balance—is used extensively in nutritional supplements, food
products, food colorings, animal feed and cosmetics and has become the most
produced algae in the world.

A Distinctively DIC Response Launching the world’s first Spirulina-derived FFC
Properly communicating evidence-based functions and product value
In commercializing PHYCONA, DIC emphasized demonstrating the product’s scientifically proven functionality and correctly communicating the commercial value that sets it apart from ordinary heath food products. The Company thus sought to obtain approval of PHYCONA as an FFC, a designation that falls under the jurisdiction of Japan’s Consumer Affairs Agency. Traditionally, labeling of food functionality in Japan had been limited to Foods for Specified Health Uses (FOSHU), which are approved by the government on a one-by-one basis, and Food with Nutrient Function Claims (FNFC) products, which comply with national standards. The FFC designation was created in 2015 with the goal of expanding choice. To be approved as an FFC, a product must comply with standards set by the government, while manufacturers must prove safety and effectiveness based on scientific evidence, establish rigorous production and quality control systems, and collect information on adverse health events.

Verifying functionality through clinical trials of the finished product
An important consideration once an application for FFC status has
been accepted is how to verify functionality. Two methods can be used:
Systematic literature review, whereby functionality is estimated based
on existing research reports, and clinical trials of the finished product,
whereby trial data is compiled into an academic paper and submitted.
DIC and DIC Lifetec chose the latter method. There are still few studies
on phycocyanin and as a result still not enough research reports to count
as scientific evidence. Accordingly, although clinical trials are more
laborious and costly than a systematic literature review, the companies
resolved that this was the best way to accurately confirm functionality
and to market PHYCONA with confidence in its performance.
In 2019, the two companies conducted a clinical trial using PHYCONA
manufactured on an experimental basis using an actual production line.
A total of 96 subjects (all female) aged 20–60 with dry skin were divided into a treatment group, which received phycocyanin, and a placebo
group, which did not. Changes in TEWL—an indicator of the skin’s
barrier function—were monitored over a period of eight weeks. Trial
results showed that the PHYCONA group saw a significant decrease in
TEWL. Trial data was compiled into an academic paper and in July 2020
the appropriate applications were submitted, resulting in PHYCONA
becoming the first FFC containing Spirulina-derived phycocyanin. In
February 2021, DIC became the first company to obtain a use patent for
“a food product containing phycocyanin as an active ingredient that has
skin moisture-improving and other beneficial properties.” A use patent
is granted for an existing substance when a previously unknown use
is discovered yielding new applications. This newest patent will enable
DIC to realize a considerable competitive advantage in the development
and sales of phycocyanin-based skin-care and related food products.
KEY PERSON from DIC
Our goal was to offer something entirely new.

It was exciting to introduce the world’s first product in the skin-care field containing an extract of Spirulina, which DIC has studied for more than 50 years. To ensure understanding of just how amazing this product really is, we recognized the need to boost general awareness of its main ingredient, phycocyanin. We are currently working to increase recognition through the media. The level of interest from both men and women in their 60s and older has been much higher than expected, underscoring the fact that proper skin care is of interest to people of all ages. Looking ahead, we will continue striving to gain a better grasp of customer needs, while at the same time expanding our operations in the healthcare field— an important goal under our current medium-term management plan—with a view to expanding into Asia, as well as into the Americas and Europe.
Product Manager, Health Care Products Group, Color Material Products Division, DIC Corporation Taro Ichimoto
Our research into skin aging led us to discover the potential of phycocyanin in this area.

The development of PHYCONA resulted from our research into skin aging, which confirmed the excellent anti-inflammatory and antioxidant properties of phycocyanin. We had to overcome a number of issues before achieving commercialization. In particular, we struggled to establish a technology for converting phycocyanin, which is sensitive to heat and alcohol, into a tablet form and coating it to make it easy for people to take while at the same time ensuring solubility and absorption. Perfecting technologies to facilitate the stable mass production of a natural material also was not easy. I feel that the acceptance of our application to have PHYCONA approved as an FFC is a refl ection of the expertise we have built up through our years of Spirulina research, as well as the extensive production technologies we have accumulated as a manufacturer of fine chemicals.
Manager, Health Care Products Business Planning & Development Group and Health Care Technical Group, Color Material Products Division, DIC Corporation Yasuyuki Imai
Special topics 2021

Employing Chemical Recycling to Realize a Closed-Loop Recycling System for Polystyrene Food Containers
Fine chemicals manufacturer DIC and food container manufacturer FPCO collaborate to implement closed-loop recycling system for polystyrene that employs chemical recycling.






Nashiji Film Made with Biomass Plastic (DIFAREN® A7440Bio)
DIC has developed a new food packaging film that is refined and visually appealing while at the same time reduces CO₂ emissions.



An FFC that Improves the Skin’s Barrier Function from the Inside Out (PHYCONA Skin Moistlifting Tablets)
DIC has developed a new “edible” skin-care product, made with phycocyanin extracted from Spirulina blue-green algae, that addresses various skin problems.