Ensuring the Safety of Chemical Substances
Goals and Achievements of Major Initiatives
・Enhance functions of comprehensive chemical substance information management systems.
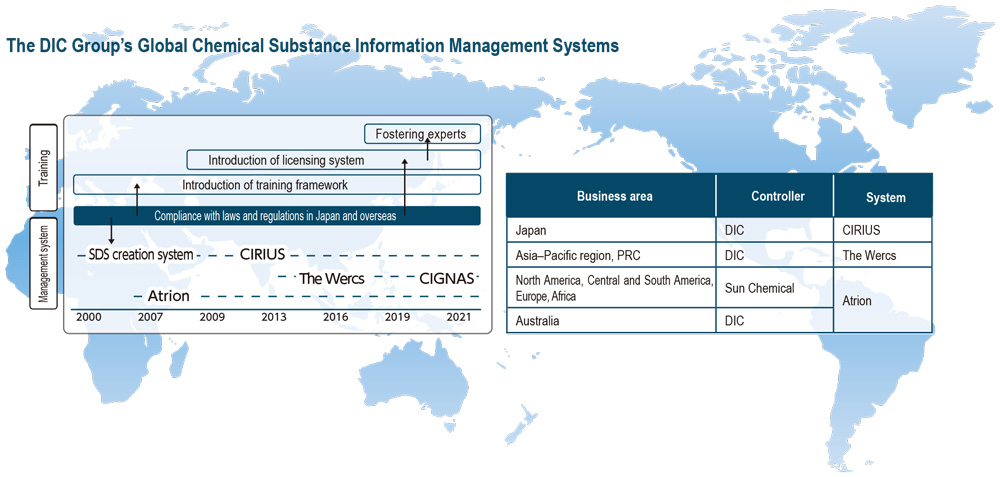
・Continue to expand deployment of the Wercs and Atrion at DIC Group companies in other countries and territories.
| Scope of target |
|
|---|---|
| Goals for fiscal year 2022 |
|
| Achievements in fiscal year 2022 |
|
| Evaluation | ★ ★★ |
| Goals for fiscal year 2023 |
|
・Review business flow to ensure compliance with laws and regulations around the world.
・Comply with laws and regulations in other countries and territories.
| Scope of target |
|
|---|---|
| Goals for fiscal year 2022 |
|
| Achievements in fiscal year 2022 |
|
| Evaluation | ★★★ ★★★ ★★★ |
| Goals for fiscal year 2023 |
|
- Evaluations are based on self-evaluations of current progress.
Key: ★★★ = Excellent; ★★ = Satisfactory; ★ = Still needs work
Basic Approach
The DIC Group continues working to provide appropriate information to stakeholders to ensure the appropriate handling of its products over their entire life cycle.
Policies and Organization
In 2002, countries and territories participating in the World Summit on Sustainable Development (WSSD) in Johannesburg, South Africa, including the United States, European Union member states and Japan, agreed on a goal for the management of chemical substances to minimize the impact thereof on human health and the environment by 2020. In 2015, the UN General Assembly set the SDGs, a collection of common goals designed as a blueprint for global society.
As a comprehensive chemicals manufacturer with operations around the world, the DIC Group created uniform standards for managing chemical substances that exceed legal and regulatory standards well before the WSSD. In line with its Environment, Safety and Health Policy (established in 1992), the Group views product stewardship* as the foundation of Responsible Care and works to provide stakeholders with information on the appropriate handling of its products over their entire life cycle. The Group also develops alternative offerings that exert less of an impact on the environment and to this end promotes the management of chemical substance information to contribute to the achievement of sustainability. To this end, the DIC Group has also established bases in the PRC and the Asia–Pacific region, better positioning it to disseminate information to Group companies around the world.
- Product stewardship is a philosophy that emphasizes assessing product-specific risks and sharing findings and information on appropriate handling with stakeholders with the aim of reducing the ESH impact of products over their entire life cycle, i.e., from the development of chemical substances through to procurement, production, transport, sale, use and disposal or recycling.
Managing Chemical Substances
In 2003, the UN Economic Commission for Europe (UNECE) issued the first edition of the GHS.*1 Many countries have since introduced the GHS, including Japan, which in 2006 compelled use of the system in the Industrial Safety and Health Act. As part of its effort to ensure effective product stewardship, the foundation of Responsible Care, DIC was early to respond to this development, providing customers with crucial hazard-related information and encouraging them to use such information to reduce risks.
Concurrent with the enforcement of the Industrial Safety and Health Act in 2006, DIC began providing GHS-compliant SDSs.*2 In 2009, the Company developed CIRIUS (Chemical Substance Information Comprehensive Management System), a proprietary system that centralizes the management of information on chemical substances in raw materials and products, as well as automatically checks various laws and regulations—including the Act on the Evaluation of Chemical Substances and Regulation of Their Manufacture, etc.—to facilitate swift responses to customers’ requests for information. In 2013, the Company began using the Wercs, an SDS and label creation system that facilitates the translation of data into 46 languages, for products destined for overseas markets, while in 2015 it also began deploying the Wercs outside Japan. At present, the system is in use at 23 Group companies in 11 countries and territories. DIC also continues to advance the creation of a new comprehensive global chemical substance information management system dubbed CIGNAS (Chemicals Information Global Network Access System). In fiscal year 2021, DIC replaced the Wercs and CIRIUS in Japan with CIGNAS. Current plans are to begin using the new system at DIC Group companies in the PRC and the Asia–Pacific region in fiscal year 2023, with the aim of achieving full deployment, replacing the Wercs, by fiscal year 2024. The Sun Chemical Group has used Atrion International Inc.’s eponymous chemical substance information management system since 2006, enabling it to provide highly accurate information to its customers worldwide.
Recognizing the importance of specialized expertise in the manufacture, import and handling of chemical substances in accordance with applicable laws and regulations, in 2000 DIC began providing related training. Since 2007, the Company has had a proprietary licensing system designed to maintain and enhance the skills of employees who have become experts in chemical substance management.
- The GHS was formally adopted by the UN in 2003 to facilitate the uniform global classification and labeling of hazard information for chemicals.
- SDSs contain information on the hazards of chemicals to ensure their safe handling.
VOICE
We participate in industry association activities and were involved in the revision of Japan’s chemical substance law.

The Responsible Care Department plays an active role in the JCIA and on behalf of DIC, a core member, we were involved in the revision of the Act on the Evaluation of Chemical Substances and Regulation of Their Manufacture, etc. Throughout this process, we struggled to cope with differences of opinion arising from the diverse perspectives of pertinent ministries, including the Ministry of Economy, Trade and Industry (METI). The situation was similar within the JCIA, with the views of individuals diverging depending on the company they represent, so it took a lot of time and effort to get everyone on the same page regarding revisions.
Efforts to meet the WSSD goal for chemical substance management will conclude in 2020. Nonetheless, initiatives aimed achieving the SDGs will continue until 2030 and discussions have already begun with the aim of developing a vision for as far in the future as 2050. As a company that handles chemical substances, we pledge to never lose sight of the need to act in a responsible manner to ensure that DIC Group can continue to play a central role in the JCIA in the years ahead.
Group Manager, Chemical Management Group, Responsible Care Department
Shinobu Yamaguchi
Creating a New Chemical Substance Information Management System and Framework
DIC’s Global Chemical Information Management Project (GCIP), established to develop the CIGNAS system, is responsible for not only system design and development but also reviewing and standardizing procedures to be used globally for gathering information to ensure smooth operation following launch. Key highlights to date include the following:
- In fiscal year 2019, project team members visited 11 DIC Group companies, in three countries to deepen their understanding of these companies' procedures for managing chemical substances. Findings were incorporated into development efforts.
- In fiscal year 2021, the project team conducted online interviews with colleagues from 12 Group companies in five countries to clarify their expectations of the new system.
Designing and Developing CIGNAS
DIC applied capabilities, experience and expertise accumulated in the design, development and operation of CIRIUS and the Wercs to designing and developing its new CIGNAS system. The Company also recognized that a unified global system for managing chemical substance information will enhance its operational efficiency and thus created a framework for data integration with its ERP system.
Individual divisions and departments make use of chemical substance information in its particular work. Accordingly, the system is used not only by experts in the management of chemical substances but also by diverse other employees across the global DIC Group. The Group was thus aware of the importance of designing the interface so that even non-experts can use CIGNAS with ease. The new system stores confidential information on, among others, the chemical composition of products and raw materials. For this reason, and because of the wide range of individuals using the system, meticulous attention to security was a key consideration in system design and development.
A Global Framework
Techniques used to manage chemical substance information vary greatly depending on country/territory and site, as does the quality of management. Given the expected further tightening of laws and regulations governing chemical substances and the increasing number and changing nature of substances used, implementing an organized global approach is essential. The DIC Group recognizes that introducing a new system is only part of the solution, and so it has also commenced efforts to establish a new information management framework to support administration of the new system after creation and deployment. In fiscal year 2019, the Chemical Substance Information Management Group was established at DIC’s corporate headquarters in Tokyo to oversee this process. In April 2020, this group also began promoting initiatives in Shanghai. In January 2023, collaboration was expanded to encompass the Asia–Pacific region, with the Chemical Substance Information Management Group in Tokyo providing appropriate support. Through such efforts, the Group will leverage know-how accumulated in Japan to integrate information management, thereby ensuring consistent quality, securing compliance and strengthening governance.
VOICE
We are working to improve customer confidence by promoting legal and regulatory compliance initiatives.

I am in charge of the GCIP at DIC (China). In fiscal year 2020, we explained the GCIP’s plan for deploying CIGNAS and the Wercs in 2022 to local Group companies (16 production facilities) on an individual basis. We are currently analyzing the documentation of each of these companies with the objective of creating an operations manual for chemical substance information management going forward.
As a legal and regulatory officer responsible for helping ensure compliance with laws and regulations governing chemical substances overseas, I gather information on laws and regulations in countries other than Japan and formulate Groupwide policies to guide the development and implementation of responses by individual companies. I try to explain complex laws and regulations, as well as to suggest appropriate responses, as simply and clearly as possible to help deepen employees’ understanding. We believe that this approach is important to gaining the confidence and trust of customers and society at large. We will continue to promote a variety of related initiatives that contribute to increased trust in the DIC Group.
Corporate ESH Department, DIC (China) Co., Ltd.
Meijing Chen
Complying with Laws and Regulations
01Complying with Laws and Regulations in Japan
DIC recognizes legal and regulatory compliance as central to risk management. In Japan, this includes fulfilling without exception obligations related to the reporting of new chemical substances set forth in the Act on the Evaluation of Chemical Substances and Regulation of Their Manufacture, etc., and the Industrial Health and Safety Act, and to the keeping of records on manufacturing, importing and sales laid out in the Poisonous and Deleterious Substances Control Act. To enhance the reliability of its compliance efforts, the DIC Group is promoting diverse initiatives, from collecting and analyzing information to formulating guidelines, promoting awareness among Group companies and customers, and advancing deployment of CIGNAS.
The Food Sanitation Act, which was amended in fiscal year 2018, stipulates the adoption of a Positive List system, which allows only substances that have been evaluated for safety to be used in utensils, containers and packaging for food. DIC manufactures a wide range of polymers, including polystyrene, inks and other raw materials used in food packaging. Accordingly, the Company is proceeding with efforts to gather information and apply for the list in cooperation with pertinent industry organizations. There were no legal violations by the DIC Group in Japan requiring the registration or reporting of chemical substances in fiscal year 2022.
VOICE
DIC and Sun Chemical are collaborating to promote a chemical substance management project.

DIC and Sun Chemical are engaged in a project to identify the criteria needed, and the framework required, for an undertaking to support a global chemical communication management system. This project seeks to define specific chemical criteria that must be utilized to assure compliance with regulations anywhere in the world. The project will involve developing an SAP integration IT platform that is seamlessly accessible by global beneficiaries. This will require an IT investment platform designed to uniformly characterize the chemical composition and safety risk of purchased materials and manufactured commercial products; a system, process, tools and discipline to continuously identify and maintain material characteristic consistencies with both chemical data and system deliverables, such as SDSs and labels; and robust support for new and onerous chemical control regulations (i.e., the U.S. EPA TSCA reset, K-REACH and Global GHS) and to accommodate international language requirements. Our objective is to provide leverage to help establish material fitness guidance with global customers and brand owners and, ultimately, to create a platform to support technological innovation and assist in the coordination of global procurement activities.
Director Global Regulatory, Sun Chemical Corporation
Robert Kendrick
Outlook for Principal Initiatives in Fiscal Year 2023
Japan’s revised Industrial Safety and Health Act will come into force in fiscal year 2023. The revised Act emphasizes the transition to a regulatory system for chemical substances based on autonomous management, notably on establishing a system for implementing autonomous management and strengthening the communication of information on hazards and toxicity. A significant number of new chemical substances will be added to the Appended Table 9 of the Order for Enforcement of the Industrial Safety and Health Act. It will also be possible to obtain SDSs without seeking approval of the other party simply by, for example, checking and scanning 2D barcodes printed on containers or viewing the appropriate website product page. The DIC Group in Japan will continue working to ensure it manages chemical substances, as well as prepares and distributes SDSs, in compliance with the revised Industrial Safety and Health Act. DIC will also take decisive steps to address the WSSD goal that will supersede the goal for 2020, which was expected to be discussed at the International Conference on Chemicals Management (ICCM) but was delayed due to COVID-19, paying close attention to how the new goal is reflected in policies, laws and regulations.
02Complying with Laws and Regulations in Other Countries and Territories
Recent years have brought the establishment and amendment of major laws and regulations governing chemical substances across East Asia. Key examples include revisions to the Republic of Korea (ROK)’s Act on the Registration and Evaluation of Chemicals (K-REACH) in fiscal year 2019 and the PRC’s China REACH legislation in fiscal year 2020. Other countries that currently do not have chemical substance registration systems, including Thailand, Vietnam, Turkey, Russia and Eurasian Economic Community member countries, are also moving in this direction, but in many cases progress is behind schedule.
Deployment of the GHS has been made mandatory in most countries, with latecomer India now taking steps toward enacting a law obliging GHS compliance. DIC gathers the latest information on chemical substances in overseas markets through local consultants, as well as through its global network, which includes Sun Chemical and other DIC Group companies, ensuring its ability to respond effectively to revisions to laws and regulations and to provide information to Group companies and customers. As a leading member of the JCIA working group charged with collecting Japanese companies’ opinions and proposals regarding the enactment and revision of laws and regulations, DIC conducts dialogue with government authorities, playing a leading role in ensuring the legal and regulatory compliance of JCIA members. Thanks to effective monitoring of regulatory trends and swift responses to revisions to pertinent laws, there were no violations of legal violations requiring the registration or reporting of chemical substances by the DIC Group in other countries and territories in fiscal year 2022.
Outlook for Principal Initiatives in Fiscal Year 2023
The DIC Group will continue pressing ahead with preparations to re-register chemical substances as required under the ROK’s revised K-REACH legislation, prioritizing substances with large volumes that are close to the re-registration deadline. The Group will also prepare to meet the re-registration deadline for Taiwan’s Toxic and Chemical Substances of Concern Control Act (TCSCCA). Additionally, the Group will continue to gather information and take steps to register chemical substances to ensure compliance with newly introduced registration systems in other countries and territories. In India, the Group will keep abreast of developments surrounding India’s move to mandate GHS compliance and will submit opinions and proposals through the JCIA.
VOICE
We gather information on laws and regulations governing chemical substances overseas with the aim of increasing public trust in the DIC Group.

In recent years, countries and territories around the world have taken steps to establish new or strengthen existing chemicals-related laws and regulations. Companies’ responses have necessarily expanded and become increasingly complex. The DIC Group, which continues to broaden its global presence, deals with a bewildering range of laws and regulations. Ensuring unfailing compliance wherever it has operations is essential for the Group to fulfill its responsibilities as a corporate citizen.
As a legal and regulatory officer responsible for helping ensure compliance with laws and regulations governing chemical substances overseas, I gather information on laws and regulations in countries other than Japan and formulate Groupwide policies to guide the development and implementation of responses by individual companies. I try to explain complex laws and regulations, as well as to suggest appropriate responses, as simply and clearly as possible to help deepen employees’ understanding. We believe that this approach is important to gaining the confidence and trust of customers and society at large. We will continue to promote a variety of related initiatives that contribute to increased trust in the DIC Group.
Manager, Chemical Management Group, Responsible Care Department, DIC Corporation
Masato Inoue
We are working to properly understand and comply with diverse laws and regulations.

DIC Korea sells products imported from other DIC Group companies. Given the increasingly diverse and specialized chemical substance laws and regulations that importers in the ROK must comply with, including K-REACH, in fiscal year 2019 we established the Import Control Team to oversee related efforts. As a part of this team, I am responsible for making certain that the chemicals we import comply with applicable laws and regulations, as well as for assisting customers in this market to do the same. Correctly understanding and implementing a wide range of laws and regulations is the team’s fundamental and most important job, and our ability to work closely with related parties to respond in a timely manner is directly linked to the Group’s profitability. We also believe it is important to inform local customers about DIC’s policy regarding managing the safety of chemicals so that they can feel secure purchasing DIC Group products. Going forward, we will continue to hone our ability to ensure legal and regulatory compliance.
Import Control Team, DIC Korea Corp.
Gu Gyo-ok
Training and Systems
In line with the principles of product stewardship, the DIC Group recognizes the importance of greater employee awareness and know-how to ongoing efforts to improve the safety of chemicals and manufactured goods. The Group places considerable emphasis on training for employees involved in the manufacture, import and handling of chemical substances in accordance with applicable laws and regulations and endeavors to improve understanding and knowledge of applicable laws and regulations in Japan and other countries and territories, which it provides through its program to foster experts and its proprietary licensing system.
01Fostering Experts
As a comprehensive global chemicals manufacturer, the DIC Group recognizes legal and regulatory compliance as central to risk management and promotes training designed to foster experts in this area. DIC began offering an entrylevel course on laws and regulations governing chemical substances in fiscal year 2014. An online format was adopted in fiscal year 2021 to make it easier for employees in the target group—mainly employees at sites with technical departments—to participate in training. In fiscal year 2022, the Company continued to provide training on the legal handling of chemical substances, expanding participation in this training to include employees of DIC Group companies. In fiscal year 2023, efforts will focus on designing courses and preparing study materials to facilitate the creation of a practical program focused on various chemicals-related laws and regulations.
02Licensing System in Japan
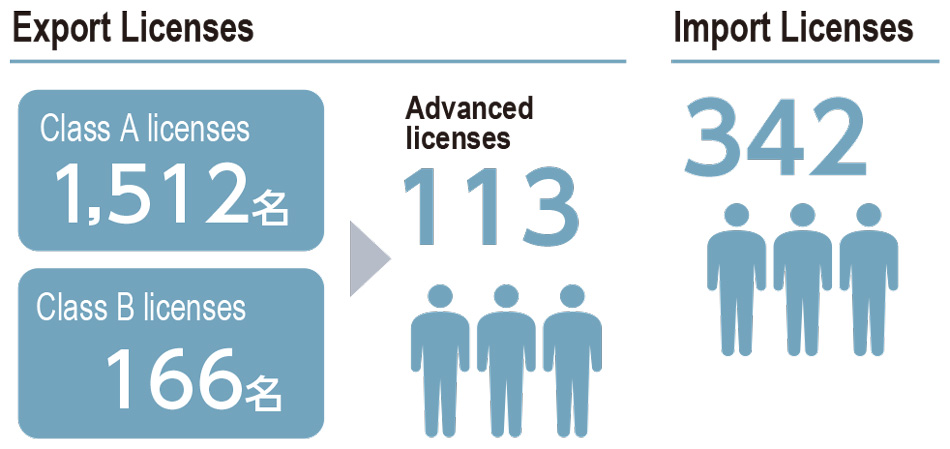
Under a proprietary licensing system, DIC provides mandatory specialized training for individuals in Japan engaged in the export and import of chemical substances and issues licenses to employees who have completed training and passed in-house examinations. The period of validity is two years for export licenses and three years for import licenses. Training for individuals involved in exporting and importing chemical substances focuses on the Foreign Exchange and Foreign Trade Act, while that for individuals involved exclusively in importing centers on the Act on the Evaluation of Chemical Substances and Regulation of Their Manufacture, etc., the Industrial Safety and Health Act and the Poisonous and Deleterious Substances Control Act. To renew a license, an employee must once again go through training and pass the in-house examination. In fiscal year 2022, training and examinations were conducted online. As of the fiscal year-end, 342 employees held an import license; 166 held a Class B export license, requiring general knowledge; and 1,512 held a Class A export license, which requires high-level specialized expertise, while a further 113 had completed an advanced export license course, an achievement requiring superior capabilities.
In fiscal year 2023, DIC will further enhance the content of this training. With the importance of economic partnership agreements increasing, owing to, among others, the entry into force of the Regional Economic Partnership (RCEP), the Company launched a licensing system for employees who prepare certificates of origin based on such agreements. DIC will also continue to expand related training in fiscal year 2023.
03Training at DIC Group Companies in Other Countries and Territories
In response to the Group’s mandate for effective management of chemical compliance, Guangdong DIC TOD Resins Co., Ltd. (TOD), which became a member of the DIC Group in July 2022, is actively promoting legal compliance efforts and is working to build a management framework. DIC (China)’s Corporate ESH Department held two presentations on managing new chemical substances for all TOD sales representatives with the goal of deepening the company’s understanding of chemical compliance management. These included explanations defining new chemical substances, legal and regulatory requirements, and the DIC Group’s compliance measures.
Looking ahead, the DIC Group will continue working to deepen chemical compliance management across the Group by providing education through presentations and training, as well as to share information on the latest legal and regulatory developments, in response to requests from Group companies in other countries and territories.

Presentation by Yundan Zhao of DIC (China)’s Corporate ESH Department on the management of new chemical substances
VOICE
This is our key consideration in designing and administering CIGNAS.

The working group responsible for designing and administering CIGNAS, of which I am a member, works continuously to enhance the efficiency and ensure the stable operation of the system. CIGNAS, which was launched in Japan in November 2021, replacing CIRIUS, is already playing a crucial role as the DIC Group’s global chemical substance information management system, including by supporting the issue of SDSs for use in other countries and territories.
Procedures for managing chemical substance information have increased and become more complex. In addition to inputting information on products and the raw materials and chemical substances used therein, we need CIGNAS to facilitate the more efficient output of information required by internal and external stakeholders. Accordingly, we continue collaborating with technical teams and Responsible Care Department colleagues to guarantee that data input and updated daily into CIGNAS is transformed swiftly into the information required for in-house chemical substance management, the provision of information to customers and other purposes. I look forward to working with everyone in optimizing this new system.
Manager, Product Safety & Regulatory Group, DIC Coporation
Hitoshi Ishizuka
We continue to provide legal compliance training.

My name is Yundan Zhao and I am in charge of chemical regulatory affairs at DIC (China). Countries around the world, increasingly concerned with sustainability, are constantly working to perfect their relevant legal and regulatory frameworks. Owing to economic globalization, amendments to chemical substance–related laws in one country may significantly impact not only that country’s chemical substance compliance management but also its exports and imports. To respond to such legal and regulatory changes, it is important to promptly secure and share pertinent information and to improve understanding on the part of all involved. This makes it possible to swiftly formulate and implement effective countermeasures.
As an officer responsible for matters pertaining to chemical laws and regulations at DIC (China), I will continue working to swiftly grasp and digest laws and regulations that require chemical substance management, conduct training and assist problem solving at all DIC Group companies in the PRC. In so doing, I hope to help lift the level of compliance management across the entire Group. I am grateful to all my colleagues for their ongoing support.
Corporate ESH Department, DIC (China) Co., Ltd.
Yundan Zhao
I will continue to provide training aimed at ensuring legal compliance.

In recent years, with awareness of issues related to health and the environment, the drive to tighten chemicals-related legislation is gathering speed worldwide. This has led to the amendment of laws and regulations. For the DIC Group, which has operations worldwide, it is crucial that we ensure access to the latest legal and regulatory information and the ability to respond promptly and decisively. To this end, it is necessary that each employee has a proper grasp of laws and regulations and that pertinent information is shared among related departments. In my capacity as a legal and regulatory officer, I will continue to promote training aimed at ensuring employees understand the relationship between DIC Group products and laws and regulations governing chemical substances that help ensure effective compliance.
Manager, Chemical Management Group, Responsible Care Department, DIC Corporation Chisato Kuriyama
We are working to expand legal compliance training.

In the modern world, legal and regulatory compliance is required across the board, from the manufacture and import of products through to sales, handling and export. A company that is unable to respond effectively cannot remain a going concern. Frameworks and systems designed to ensure compliance are important, but compliance cannot be achieved without the understanding of each and every employee of the DIC Group. The Group’s product portfolio is extensive, so the number of laws and regulations with which we must comply is considerable. We currently provide wide-ranging training regarding laws and regulations governing chemical substances. As one of the people in charge of this training, I will work to step up training to better respond to needs pertaining to employees and products to contribute to increasingly effective compliance.
Manager, Chemical Management Group, Responsible Care Department, DIC Corporation Hirofumi Higashino
Position on the Use of Animals in Testing
In line with the “3Rs” of animal use in research (replacement, reduction, refinement), which are guidelines designed to ensure the more ethical use of animals in testing, the DIC Group actively promotes safety evaluation using quantitative structure–activity relationship (QSAR) models that do not employ animals.
Safe Product Transport
The DIC Group has created Yellow Cards containing simplified SDSs. This provides critical information to transport personnel, facilitating the appropriate responses in the unlikely event of an accident to protect the environment and ensure safety.

